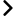
September 17, 2020

July 19, 2018
June 13, 2018

February 06, 2018
January 17, 2018
August 24, 2017
May 25, 2017

March 03, 2017
February 13, 2017
June 30, 2016
April 28, 2016
March 08, 2016
January 29, 2016
October 28, 2015
September 24, 2015
May 12, 2015
February 02, 2015
December 11, 2014
October 09, 2014
June 18, 2014
May 07, 2014
March 06, 2014
February 19, 2014
October 17, 2013
August 02, 2013
June 25, 2013
May 30, 2013
April 30, 2013
March 14, 2013
November 30, 2012
October 30, 2012
August 06, 2012
June 01, 2012
May 01, 2012
March 01, 2012
February 01, 2012
January 01, 2012
November 01, 2011
October 01, 2011
August 01, 2011
July 01, 2011
June 01, 2011
May 01, 2011