By Jayla Burton, Program Manager
When I attended my first San Antonio Breast Cancer Symposium (SABCS) conference back in 2019, I was galvanized by the strong presence of patient advocates, yet shocked at how many of the sessions lacked patient-centered representation. At that very first moment, I understood why it was important for Breast Cancer Action to translate the science, uplift the patient narrative, and pose critical questions in our unique role as the breast cancer industry watchdog each year.
I looked forward to returning to San Antonio the following year with an idea of what to expect—but what I didn’t expect was having to attend the symposium virtually for two consecutive years.
This year the symposium was held both in-person and virtually. Our Executive Director, Dr. Krystal Redman (KR) and I made the conscious decision to tune into the virtual platform to hear breast cancer doctors, researchers, and scientists from around the world meet, share, and review the latest science.
Many themes emerged this year, including the change in the continuum of care due to COVID-19, race and disparities, trust in our healthcare systems, emerging therapies for Triple Negative Breast Cancer (TNBC) and so much more. Hopefully you had the opportunity to stay engaged with us on social media throughout the week as we did our rapid response. However, if you weren’t able to tune in throughout this week, Krystal and I will recap on our main takeaway from the symposium and highlight connections to our programmatic work over the last year.
Earlier this year we called on President Joe Biden and his administration to address the breast cancer crisis by increasing transparency and accountability in regulatory and approval processes within agencies such as the Food and Drug Administration (FDA). More recently, we were critical of the FDA’s implementation of black box warning labels and informed consent for those considering breast implants. So, when I saw the session titled, Regulatory Insights to the 2021 Early-Stage Breast Cancer Approvals presented on day two, I added it to my hefty itinerary.
Over the past ten years, there have been six FDA approvals in the neoadjuvant and adjuvant setting.
Definitions:
Neoadjuvant chemotherapy is delivered before surgery with the goal of shrinking a tumor or stopping the spread of cancer, to make surgery less invasive and more effective. Adjuvant therapy is administered after surgery to get rid of remaining cancer cells and reduce the chance of reoccurrence.
ER+: Estrogen positive breast cancer
HER2-: HER2-positive breast cancer is a breast cancer that tests positive for a protein called human epidermal growth factor receptor 2
Ki67 score: a score used to measure the percentage of positively stained tumor cells among the total number of malignant cells
This regulatory session consisted of FDA scientists and representatives who talked about two FDA approvals for early breast cancer that occurred in 2021: Pembrolizumab (also known as Keytruda) for the treatment of patients with high risk, early stage triple negative breast cancer in combination with chemotherapy as neoadjuvant treatment, and continued as an adjuvant treatment after surgery; and Abemaciclib in combination with endocrine therapy, tamoxifen, or an aromatase inhibitor for the adjuvant treatment of adult patients with ER+ HER2- node positive early breast cancer at high risk of recurrence and a Ki67 score of greater to or equal to 20%.
When it comes to the FDA approval of breast cancer treatments and/or devices we have always asked these critical questions: Does this treatment extend overall survival (OS)? Does the treatment improve quality of life? And does this cost less than therapies that are already available?
Pembrolizumab is the first approval for an immunotherapy in the early breast cancer setting, and the first time a single trial design was employed to obtain approval in the neoadjuvant and adjuvant settings. This drug was covered in one of our blogs from last year’s symposium while the treatment awaited FDA approval.
Pembrolizumab was first introduced back in 2014 and was denied accelerated approval during the third stage of the drugs analysis, (Intermin Analysis 3) the fourth stage of analysis (Intermin Analysis 4), the FDA approved the drug for patients with high-risk early stage Triple Negative Breast Cancer based on data from the KEYNOTE-522 study published on July 27, 2021. Triple Negative Breast Cancer is an aggressive type of breast cancer that characteristically has a high recurrence rate within the first five years after diagnosis, and is most common in younger women and Black women.
One of the endpoints measured in the KEYNOTE-522 study was event-free survival (EFS), which is the length of time after primary treatment for a cancer that the patient remains free of certain complications or events that the treatment was intended to prevent or delay, including the return of the cancer. The other primary endpoint was pathologic complete response (pCR), which refers to the absence of invasive/in situ cancer in the breast and/or axillary lymph nodes. Overall survival was a key secondary endpoint.
Dr. Mirat Shah’s presentation, Pembrolizumab for high-risk early-stage triple negative breast cancer, discussed how at Interim Analysis 4 the EFS endpoint was met (HR=0.63 [95% CI, 0.48-0.82]; p=0.00031). However, at Interim Analysis 4, the overall survival data was immature and the endpoint was not met (HR=.072 [95% CI, 0.51-1.02]), and needed further analysis.
Additionally, Dr. Shah reported on some important safety findings that I think are crucial to take into consideration. She reported that most of the added toxicity from neoadjuvant and adjuvant Pembrolizumab comes from immune mediated adverse reactions. Forty-three percent of patients on Pembrolizumab experienced adverse event including increased hospitalizations, dose discontinuation, and dose interruptions. The most common immune-mediated adverse reactions included infusion reactions (18%), hypothyroidism (15%), severe skin reactions (6%), hyperthyroidism (5%), and adrenal insufficiency (3%).
Let’s turn back to our critical questions:
Does Pembrolizumab treatment extend overall survival? KEYNOTE-522 showed that Pembrolizumab in combination with chemotherapy before surgery and continued as a single agent after surgery significantly prolonged event-free survival (EFS) versus the placebo group that only received chemotherapy. However, this does not indicate whether or not it improves overall survival.
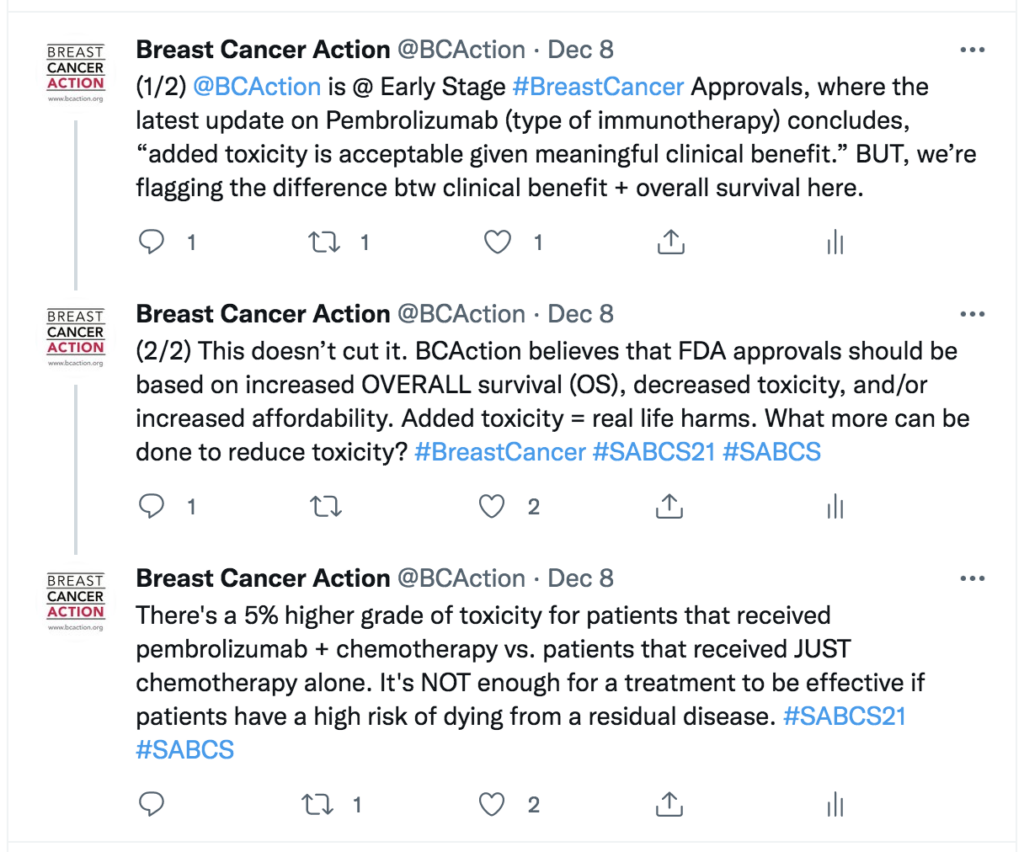 Does Pembrolizumab the treatment improve quality of life? Dr. Shah closed out her presentation saying the “…added toxicity is acceptable given meaningful clinical benefit.” But we know added toxicity means real life harms, and more needs to be done to reduce toxicity in order to improve patients’ quality of life.
Does Pembrolizumab the treatment improve quality of life? Dr. Shah closed out her presentation saying the “…added toxicity is acceptable given meaningful clinical benefit.” But we know added toxicity means real life harms, and more needs to be done to reduce toxicity in order to improve patients’ quality of life.
And finally, does Pembrolizumab cost less than therapies that are already available? This wasn’t covered during Dr. Shah’s presentation, so I had to do some digging on my own. Pembrolizumab costs about $12,500 a month, or $150,000 a year.
Yes, you read that right. $150,000 a year.
So in addition to the physical toxicity stated above, the majority of TNBC patients are young and Black, and already experience additional barriers to getting the care they need, now have to navigate the huge financial burden for a treatment that doesn’t even improve overall survival.
At Breast Cancer Action, we think people living with breast cancer deserve better.